Bristol-Myers Squibb Company announced results from CheckMate -025, a Phase 3 clinical study comparing BMS’s drug Opdivo (nivolumab) to everolimus in advanced renal cell carcinoma (RCC) after prior anti-angiogenic treatment. The CheckMate study findings to date show a significant overall survival (OS) benefit for Opdivo, which in the trial demonstrated a median OS benefit of 25 months compared to 19.6 months for everolimus. Clinical benefit for Opdivo was observed regardless of level of PD-L1 expression. The safety profile shown in CheckMate -025 is consistent with previously reported Opdivo trials. These data was presented Saturday, September 26, during the 2015 European Cancer Congress (ECC2015) at Vienna, Austria. The trial results were also featured, supported by Bristol-Myers Squibb, during the ECC2015 press program on September 25 and have been published in a paper in The New England Journal of Medicine (NEJM), representing the ninth publication in the NEJM for Opdivo.
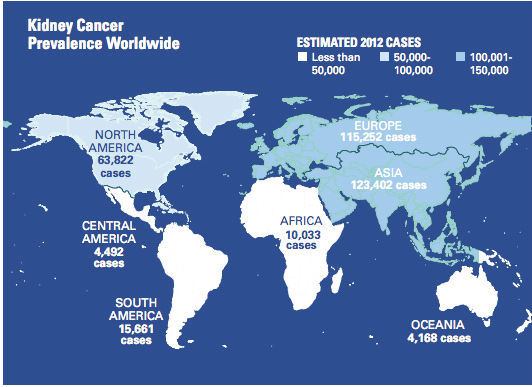
Kidney cancer is the 13th most common cancer worldwide with approximately 338,000 cases in 2012. Incidence rates vary substantially worldwide with generally high rates in
Europe, North America and Western Pacific regions. In the United States, there are approximately 62,000 new cases and about 4,000 deaths from RCC estimated for 2015.
Europe recorded 115,252 cases in 2012, and Asia: 123,402 cases in that year.
Graphic courtesy Bristol-Myers Squibb
Globally, the five-year survival rate for those diagnosed with advanced kidney cancer is 12.1%. The five-year survival rate for kidney cancer that has spread beyond the kidney (Stage IV) is only eight percent in the United States and 10 percent in the UK.
Renal cell carcinoma, or RCC, the most common type of kidney cancer in adults, accounts for about nine out of 10 kidney cancers in the United States and more than 100,000 deaths worldwide each year. Clear-cell RCC is the most prevalent type of RCC and constitutes 80% to 90% of all cases., with the highest rates of the disease found in North America and Europe.
Typically, RCC forms as a single tumor in the kidney, but more than one tumor may grow in one or both kidneys. There are several subtypes of RCC including:
• Clear cell renal cell carcinoma – the most common type of RCC, accounting for about 7 out of 10 patients; when viewed under a microscope, the cancer cells appear clear.
• Papillary renal cell carcinoma – approximately 10 percent of people with RCC have this type of disease; when viewed under a microscope, the cancer cells appear pink and finger-like.
• Chromophobe renal cell carcinoma – fewer people with RCC have this type of the disease (about 5 in 100 patients); when viewed under a microscope, the cancer cells look like clear cell RCC but larger.
• The remaining subtypes are very rare, each making up less than one percent of RCCs:
· Collecting duct RCC
· Multilocular cystic RCC
· Medullary carcinoma
· Mucinous tubular and spindle cell carcinoma
· Neuroblastoma-associated RCC
Though unlikely, the disease can also be labeled as unclassified renal cell carcinoma if the characteristics of the cancer cells do not fit within the above descriptions.
A number of factors may increase a person’s likelihood of developing RCC, such as:
• Smoking – increased risk appears to be related to how much a person smokes
• Obesity – obesity may cause changes in certain hormones that can lead to RCC
• Exposure to certain substances such as asbestos,
the metal cadmium and some herbicides
• Family history of kidney cancer; certain genetic
conditions
• High blood pressure (Hypertension)
• Gender–Men are twice as likely to develop RCC as women
• Advanced Kidney Disease — especially patients needing dialysis
• Race – African Americans and American Indians/ Alaska natives are at a higher risk than whites
The NEJM paper, entitled “Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma” (September 25, 2015DOI: 10.1056/NEJMoa1510665) is coauthored by Robert J. Motzer, M.D., Bernard Escudier, M.D., David F. McDermott, M.D., Saby George, M.D., Hans J. Hammers, M.D., Ph.D., Sandhya Srinivas, M.D., Scott S. Tykodi, M.D., Ph.D., Jeffrey A. Sosman, M.D., Giuseppe Procopio, M.D., Elizabeth R. Plimack, M.D., Daniel Castellano, M.D., Toni K. Choueiri, M.D., Howard Gurney, M.D., Frede Donskov, M.D., Ph.D., Petri Bono, M.D., Ph.D., John Wagstaff, M.D., Thomas C. Gauler, M.D., Takeshi Ueda, M.D., Ph.D., Yoshihiko Tomita, M.D., Fabio A. Schutz, M.D., Christian Kollmannsberger, M.D., James Larkin, M.D., Ph.D., Alain Ravaud, M.D., Ph.D., Jason S. Simon, Ph.D., Li-An Xu, Ph.D., Ian M. Waxman, M.D., and Padmanee Sharma, M.D., Ph.D. for the CheckMate 025 clinical trial Investigators.
The coauthors note that the CheckMate 025 randomized, open-label, phase 3 study has associated Nivolumab, a programmed death 1 (PD-1) checkpoint inhibitor, with encouraging overall survival in uncontrolled studies involving previously treated patients with advanced renal-cell carcinoma. This compared nivolumab with everolimus in patients with renal-cell carcinoma who had received previous treatment. The CheckMate 025 clinical trial (ClinicalTrials.gov number, NCT01668784)is funded by Bristol-Myers Squibb.
A total of 821 patients with advanced clear-cell renal-cell carcinoma for which they had received previous treatment with one or two regimens of antiangiogenic therapy were enrolled in the trial and randomly assigned (in a 1:1 ratio) to receive 3 mg of nivolumab per kilogram of body weight intravenously every two weeks or a 10-mg everolimus tablet orally once daily. The trial’s primary end point was overall survival. Secondary end points included the objective response rate and safety.
The median overall survival was 25.0 months (95% confidence interval [CI], 21.8 to not estimable) with nivolumab and 19.6 months (95% CI, 17.6 to 23.1) with everolimus. The hazard ratio for death with nivolumab versus everolimus was 0.73 (98.5% CI, 0.57 to 0.93; P=0.002), which met the prespecified criterion for superiority (P0.0148). Grade 3 or 4 treatment-related adverse events occurred in 19% of the patients receiving nivolumab and in 37% of the patients receiving everolimus; the most common event with nivolumab was fatigue (in 2% of the patients), and the most common event with everolimus was anemia (in 8%).
The investigators conclude that among patients with previously treated advanced renal-cell carcinoma, overall survival was longer and fewer grade 3 or 4 adverse events occurred with nivolumab than with everolimus. This study also showed a higher number of objective responses with nivolumab than with everolimus, many of which were durable. Median progression-free survival was similar in the two treatment groups and was consistent with that reported in an uncontrolled study involving patients who had previously received antiangiogenic therapy. Moreover, the results of a comparison of progression-free survival between the nivolumab group and the everolimus group suggest that progression-free survival was not a surrogate for overall survival in this study. The late separation of the progression-free survival curves suggested a potential delayed benefit in progression-free survival with nivolumab.
The researchers note that this delayed benefit was subsequently quantified in a sensitivity analysis that included patients who had not had disease progression or died at 6 months; the median progression-free survival was longer with nivolumab than with everolimus in this subgroup of patients, and suggest that these patients probably contributed to the overall survival benefit that was observed with nivolumab in this study. They report that they consistently observed prolonged survival with nivolumab, as compared with everolimus.
The full text of the NEJM paper is available at NEJM.org.
 “Patients with advanced renal cell carcinoma are in need of new treatment approaches that provide improved survival, safety and tolerability Patients with advanced renal cell carcinoma are in need of new treatment approaches that provide improved survival, safety and tolerability,” says Robert J. Motzer, M.D. , a medical oncologist at Memorial Sloan Kettering Cancer Center In New York City and lead author of the NEJM paper. “This is the first Phase 3 study to demonstrate the efficacy of an immune checkpoint inhibitor in advanced renal cell carcinoma. The results show meaningful clinical benefit with Opdivo treatment, producing a significant overall survival advantage and greater number of objective responses compared to everolimus, a current standard of care in the treatment of advanced kidney cancer.”
“Patients with advanced renal cell carcinoma are in need of new treatment approaches that provide improved survival, safety and tolerability Patients with advanced renal cell carcinoma are in need of new treatment approaches that provide improved survival, safety and tolerability,” says Robert J. Motzer, M.D. , a medical oncologist at Memorial Sloan Kettering Cancer Center In New York City and lead author of the NEJM paper. “This is the first Phase 3 study to demonstrate the efficacy of an immune checkpoint inhibitor in advanced renal cell carcinoma. The results show meaningful clinical benefit with Opdivo treatment, producing a significant overall survival advantage and greater number of objective responses compared to everolimus, a current standard of care in the treatment of advanced kidney cancer.”
Approximately 30 percent of patients with RCC present with metastatic or advanced disease at diagnosis. Despite multiple available treatment approaches for advanced RCC, available second-line therapies are associated with limited OS, and significant toxicities and limitations in tolerability, with the majority of current treatment options providing modest progression-free survival benefit.
 “We continue to see the potential of our Immuno-Oncology agent, Opdivo, to provide meaningful improvement in multiple tumor types over current standards of care in terms of overall survival,” says Michael Giordano, Senior Vice President and Head of Oncology Development at Bristol-Myers Squibb (BMS) accountable to the Chief Scientific Officer. “Results of CheckMate -025 show that Opdivo has a significant survival advantage over standard of care in patients with advanced kidney cancer who have progressed following prior treatment. These data also reinforce our Immuno-Oncology research goal to provide patients with long-term survival, and brings further confidence to the approach taken in our broader RCC development program, including the combination of Immuno-Oncology agents.”
“We continue to see the potential of our Immuno-Oncology agent, Opdivo, to provide meaningful improvement in multiple tumor types over current standards of care in terms of overall survival,” says Michael Giordano, Senior Vice President and Head of Oncology Development at Bristol-Myers Squibb (BMS) accountable to the Chief Scientific Officer. “Results of CheckMate -025 show that Opdivo has a significant survival advantage over standard of care in patients with advanced kidney cancer who have progressed following prior treatment. These data also reinforce our Immuno-Oncology research goal to provide patients with long-term survival, and brings further confidence to the approach taken in our broader RCC development program, including the combination of Immuno-Oncology agents.”
CheckMate -025 was stopped in July because an assessment conducted by the independent Data Monitoring Committee (DMC) concluded that the study met its primary endpoint, demonstrating superior OS in patients receiving Opdivo compared to the control arm. Opdivo was granted Breakthrough Therapy Designation for advanced RCC by the U.S. Food and Drug Administration based on results from this trial and the clinical need for additional treatment approaches for RCC. Breakthrough Therapy designation is an FDA program intended to expedite the development and review of medicines with early signals of potential clinical benefit in serious diseases to help ensure patients have access to new therapies as soon as possible.
CheckMate -025 marks the first time any agent has demonstrated, as a primary endpoint, an overall survival benefit vs. standard of care in second-line metastatic renal cell carcinoma. Bristol-Myers Squibb has a broad, global development program to study Opdivo in multiple tumor types consisting of more than 50 trials as monotherapy or in combination with other therapies in which more than 8,000 patients have been enrolled worldwide. Opdivo, a programmed death-1 (PD-1) immune checkpoint inhibitor, has received approval from the FDA as a monotherapy in two cancer indications. Opdivo became the first PD-1 immune checkpoint inhibitor to receive regulatory approval anywhere in the world on July 4, 2014 when Ono Pharmaceutical Co. announced that it received manufacturing and marketing approval in Japan for the treatment of patients with unresectable melanoma. In the U.S., the Food and Drug Administration (FDA) granted its first approval for Opdivo for the treatment of patients with unresectable or metastatic melanoma and disease progression following Yervoy (ipilimumab) and, if BRAF V600 mutation positive, a BRAF inhibitor.
“Results from CheckMate -025 mark the third tumor in which Opdivo has shown an overall survival benefit in a Phase 3 trial” Michael Giordano notes. “The Breakthrough Therapy Designation in advanced renal cell carcinoma is a clear signal of the need for additional treatment approaches for RCC and reflects part of our broad commitment to Immuno-Oncology research that may address many types of advanced cancers.”
This is the fourth Breakthrough Therapy Designation granted for Opdivo by the FDA, with previous indications including patients with Hodgkin lymphoma after failure of autologous stem cell transplant and brentuximab, previously treated advanced melanoma, and previously treated non-squamous non-small cell lung cancer.
Bristol-Myers Squibb has a broad, global development program to study Opdivo in multiple tumor types consisting of more than 50 trials as monotherapy or in combination with other therapies in which more than 8,000 patients have been enrolled worldwide.
Sources:
Bristol-Myers Squibb
The New England Journal of Medicine (NEJM)