Researchers at The University of Texas MD Anderson Cancer Center have found a new function for the p53 gene regulating the immune system’s response to lung cancer via its interaction with the PDL1 protein. Their study, entitled “PDL1 Regulation by p53 via miR-34,” was published in the Journal of the National Cancer Institute.
p53 is a crucial gene to target against cancer, as it is one of the most important tumor-supressor genes and is found mutated in 42 percent of all common cancers. In lung cancer, however, the mutation rate of p53 rises to 70 percent. A fully functional p53 gene instructs damaged or abnormal cells either to repair themselves or to commit “suicide,” an induced cell-death program called apoptosis, whenever the damage is too severe.
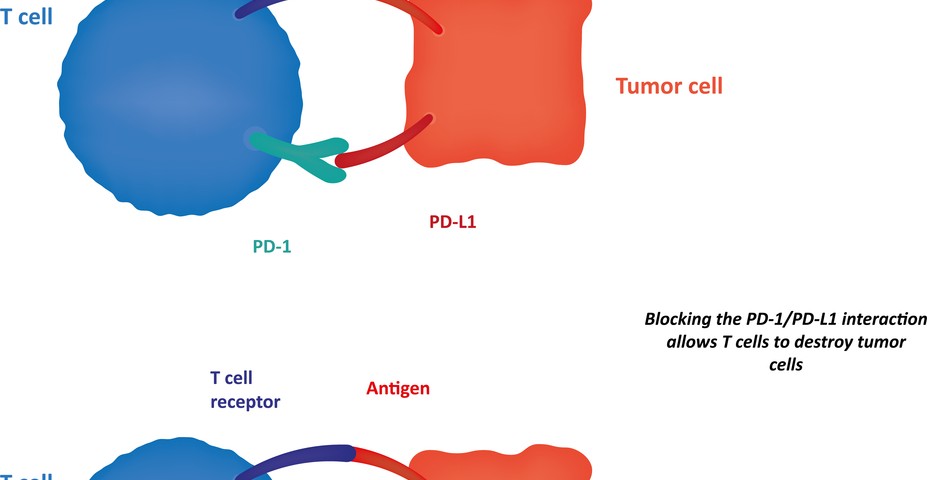
In this study, the team of scientists discovered that p53 blocks the programmed death-ligand 1 (PDL1) protein, an important player responsible for shutting down the immune system’s protective response to cancer cells. Specifically, PDL1 binds to its receptor, the programmed cell death protein 1 (PD-1), located at the surface of T cells, triggering a signaling cascade that inhibits T cell proliferation and activation. Additionally, researchers observed that p53 modulates PDL1 by activating microRNA (miR)-34a (a class of special RNA molecules that regulate gene activity).
“The interaction is specific: p53 activates the micro RNA miR-34a, which in turn directly blocks expression of PDL1,” first author Maria Angelica Cortez, PhD, instructor of Experimental Radiation Oncology, said in a press release. “If you lose p53 function, then miR-34a is lost and PDL1 is over-expressed.”
The team used cell lines and tumor samples from non-small cell lung cancer (NSCLC) patients (with mutated p53 versus functional p53 tumors) and a mouse model for human NSCLC. When mice were treated with miR-34a, loaded in vesicles called liposomes (MRX34), either alone or in combination with radiotherapy, they observed MRX34 significantly reduced PDL1 expression, preventing T cell inhibition. MRX34, a first-in-class miR-34-based cancer therapy being developed by Mirna Therapeutics in Austin, Texas, is in Phase 1 clinical trials for several type of oncologic malignancies at MD Anderson and other cancer clinics.
The results revealed that in NSCLC patients, expression of p53 and PDL1 were inversely correlated and that tumors with p53 mutated had increased levels of PDL1 and lower levels of miR-34a. Moreover, patients with low PDL1 and high p53 expression or with high p53 and miR-34a levels had longer median survival when compared to those with low expression of p53 and miR-34a and higher PDL1. When the team engineered NSCLC cell lines to express miR-34a, PDL1 expression was suppressed. Moreover, when MRX34 was injected into mice tumors, the levels of miR-34a were increased while PDL1 expression was significantly reduced. Importantly, upon radiation therapy, MRX34-treated animals had increased number of T cells infiltrating the tumor microenvironment and decreased tumor growth.
James Welsh, MD, associate professor of Radiation Oncology at MD Anderson and study lead author, commented, “Identifying this role for tumor-suppressing p53 provides both a potential biomarker for response to important new cancer immunotherapy drugs and a possible new therapeutic pathway for treatment.”