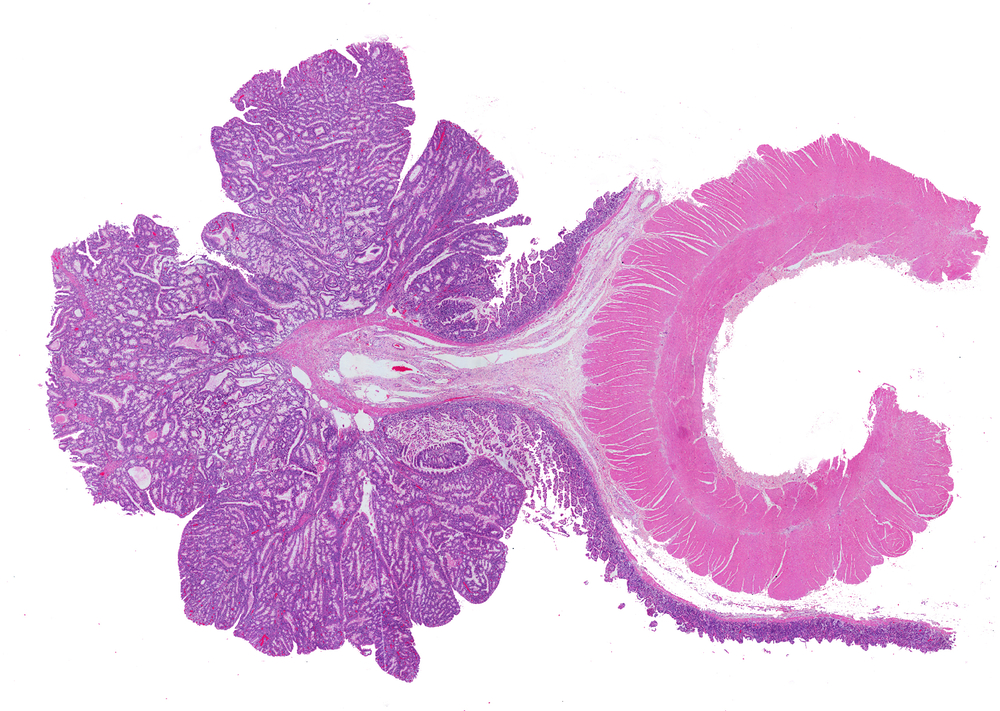
 A “striking” new study that made the cover of the current Cancer Research journal identified an oral biologic medication that successfully treats chronic, precancerous inflammation in the intestine of an animal model of precancerous polyps. The work was conducted at University at Buffalo School of Medicine’s Biomedical Science program by MD/PhD student Allen Chung in the laboratory of Nejat Egilmez, PhD, who is now at the University of Louisville.
A “striking” new study that made the cover of the current Cancer Research journal identified an oral biologic medication that successfully treats chronic, precancerous inflammation in the intestine of an animal model of precancerous polyps. The work was conducted at University at Buffalo School of Medicine’s Biomedical Science program by MD/PhD student Allen Chung in the laboratory of Nejat Egilmez, PhD, who is now at the University of Louisville.
“Our most important finding is that disease-promoting inflammatory cells in the mouse intestine can be targeted by oral formulations of purified cellular proteins, rendering the inflammatory cells less able to cause disease,” said Dr. Chung in a news release from the university.
Chung’s paper, “Oral Interleukin-10 Alleviates Polyposis via Neutralization of Pathogenic T-Regulatory Cells,” was the first to demonstrate treatment with an oral biologic of a special genetically-induced form of colon cancer. Biologics, or drug molecules derived from an organism, are usually administered by an injection, but Chung developed an orally-administered form that did not act before reaching the intestinal surface.
“We found that high pharmacologic levels of the bioactive drug of interest can be achieved at the intestinal surface without systemic circulation of the drug,” explained Dr. Chung. In this way, kidney, liver, and brain toxicities due to the drug can be minimized.
To prevent systemic delivery of the biologic, the team used polymer micro-particles that Chung’s collaborators at Brown University developed. “Site-specific oral therapy for intestinal disorders is a promising avenue of research,” commented Dr. Chung.
The biologic of choice was interleukin-10, an immuno-modulatory compound that occurs naturally in the body. When the team delivered interleukin-10 to the intestines of mice with precancerous polyps, interleukin-10 acted to reverse the “rewiring” of immune cells that was causing the cells to contribute to inflammation. As a result, the mice were relieved of symptoms that included anemia, enlarged spleen, and weight loss.
The treatment will be most relevant for individuals over the age of 50 years, a time at which polyps typically arise. Persons over 60 years old have a 25% chance of developing polyps, which can be either benign or cancerous. Rather than undergo the standard surgical removal process, patients may be able to use this new drug after it goes through clinical testing and Food and Drug Administration approval.
For Chung, the research is the basis of his thesis. Dr. Chung’s project was sparked by an interest in the relationship between inflammation and polyp formation. He began to investigate what role immunologic activity in the intestine played in polyposis. “It has long been known that inflammation within the colon increases the risk of developing colon cancer,” stated Dr. Chung.
Dr. Chung’s hard work may have implications beyond polyposis. “We are now involved in studies which hope to determine some of the immunologic phenomena we observed in our mouse models are representative of intestinal disease in human patients who harbor genetic mutations which predispose them to develop colon cancer,” Dr. Chung concluded. Individuals with Crohn’s disease or ulcerative colitis are at a high chance of developing colon cancer.