Dr. John X.J. Zhang, PhD and his team of bioengineers from the Thayer School of Engineering at the private Ivy League research university Dartmouth College in Hanover, New Hampshire have demonstrated a novel system in which nano-engineered particles are coupled with microfluidic chips in order to capture and manipulate circulating tumor cells (CTCs).
This ability to quantify rare tumor markers will enable oncologists to make more precise prognoses and to select the most appropriate therapies for a particular case. The microscale immunoassays can also be further interfaced with fluorescent microscopy to yield cancer cell imaging.
The investigators’ Open Source research paper, entitled “Microscale Magnetic Field Modulation for Enhanced Capture and Distribution of Rare Circulating Tumor Cells,” (Scientific Reports 5, Article number: 8745 Published 04 March 2015 doi:10.1038/srep08745), has been published in the journal Scientific Reports, coauthored by Peng Chen of the University of Texas at Austin Department of Biomedical Engineering; Kazunori Hoshino of the University of Connecticut Department of Biomedical Engineering at Storrs, CT; and Yu-Yen Huang and John X.J. Zhang of Dartmouth College’s Thayer School of Engineering.
Dr. Zhang’s team focused on developing a new interface between living cells and hybrid microsystems, in turn enabling rigorous design, modeling, manufacturing, and validation of high-performance and widely deployable bio-analytical microsystems for point-of-care and globally-relevant diagnostic applications.
The coauthors note that the immunomagnetic assay combines the powers of the magnetic separation and of biomarker recognition, and has been shown to be an effective tool with which to perform rare Circulating Tumor Cells detection. Key factors associated with immunomagnetic assay include the capture rate, which indicates the sensitivity of the system, and distributions of target cells after capture, which impact the cell integrity and other biological properties critical to downstream analyses.
The research team has demonstrated that the benefits of immunomagnetic assay can be effectively combined with microfluidic technology to provide high-throughput CTC screening. Resulting high sensitivity assays yield increased cell capture rates and reduced cell aggregation making them suitable for downstream CTC analyses at the single cell level.
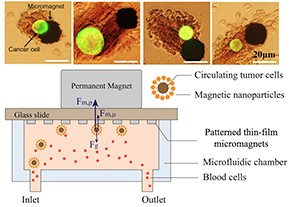 In their paper they present a theoretical framework and technical approach for implementing microscale magnetic immunoassay through modulation of local magnetic field towards enhanced capture and distribution of rare cancer cells. Through the design of a two-dimensional micromagnet array, the investigators characterize the magnetic field generation and quantify the micromagnets’ impact on rare cell separation, observing that “Good agreement is achieved between the theory and experiments using a human colon cancer cell line (COLO205) as the capture targets.”
In their paper they present a theoretical framework and technical approach for implementing microscale magnetic immunoassay through modulation of local magnetic field towards enhanced capture and distribution of rare cancer cells. Through the design of a two-dimensional micromagnet array, the investigators characterize the magnetic field generation and quantify the micromagnets’ impact on rare cell separation, observing that “Good agreement is achieved between the theory and experiments using a human colon cancer cell line (COLO205) as the capture targets.”
The researchers point out that rare cell separation has been an important emerging process towards early diagnosis of diseases such as cancer, and that in particular, “Circulating Tumor Cells (CTCs), referring to the cells that have shed into the vasculature from a primary tumor site, and circulate in the bloodstream, have been demonstrated to be clinically significant due to its values in cancer diagnosis, prognosis and treatment monitoring.” In the paper, they demonstrate the enhanced capture and distribution for CTCs detection by modulating the surface magnetic field with low-profile microscale magnetic structures.
 “This project demonstrates that a relatively simple blood test may eventually be able to provide unambiguous information to doctors about particular cancers in individuals,” says Dr. Zhang in a Dartmouth release, which notes that live cells provide a vital model systems for studying organism development as it relates to human disease. Because invasive cancers shed tumor cells into the blood, the ability to detect those cells at an early stage will enable physicians to more accurately determine a patient’s prognosis and select the best therapy alternatives. The scientists envision that being able to capture and immunophenotype CTCs shed by early stage cancers will revolutionize cancer risk assessment, treatment selection, response monitoring, and development of novel therapies.
“This project demonstrates that a relatively simple blood test may eventually be able to provide unambiguous information to doctors about particular cancers in individuals,” says Dr. Zhang in a Dartmouth release, which notes that live cells provide a vital model systems for studying organism development as it relates to human disease. Because invasive cancers shed tumor cells into the blood, the ability to detect those cells at an early stage will enable physicians to more accurately determine a patient’s prognosis and select the best therapy alternatives. The scientists envision that being able to capture and immunophenotype CTCs shed by early stage cancers will revolutionize cancer risk assessment, treatment selection, response monitoring, and development of novel therapies.
“The concept is to use novel cell-machine interfaces, integrated sensing, actuation and biomarker recognition functionalities to isolate these rare cells (1 per 109 hematologic cells) from whole blood to determine malignancy unambiguously,” Dr. Zhang explains. “We will base the quantitative assessment on multiple tumor markers.”
Dr. Zhang’s goal is to transition this cutting-edge technology from laboratory to clinic, enabling physicians to diagnose and manage cancer by way of simple blood tests, and he projects that this technology has potential to increase cure rates for cancers like breast cancer.
Dr. Zhang is a professor at Dartmouth’s Thayer School of Engineering, and his cancer research is facilitated by Dartmouth’s Dartmouth-Hitchcock Norris Cotton Cancer Center where he is a Member of the Cancer Imaging & Radiobiology Research Program. The Norris Cotton Cancer Center combines advanced cancer research at Dartmouth and the Geisel School of Medicine with patient-centered cancer care provided at Dartmouth-Hitchcock Medical Center in Lebanon, NH, at Dartmouth-Hitchcock regional locations in Manchester, Nashua, and Keene, NH, and St. Johnsbury, VT, and at 12 partner hospitals throughout New Hampshire and Vermont.
 On March 26, the National Cancer Institute (NCI) announced that it is renewing its Cancer Center Support Grant to Norris Cotton Cancer Center, continuing NCCC’s designation as a “Comprehensive Cancer Center” — the top designation bestowed by NCI for the highest quality patient care, cancer research, cancer prevention, and education of oncologists and researchers. NCCC is one of only 41 NCI-designated Comprehensive Cancer Centers in the United States and is the only such center in New Hampshire or Vermont. To learn more about Norris Cotton Cancer Center research, programs, and clinical trials, visit:
On March 26, the National Cancer Institute (NCI) announced that it is renewing its Cancer Center Support Grant to Norris Cotton Cancer Center, continuing NCCC’s designation as a “Comprehensive Cancer Center” — the top designation bestowed by NCI for the highest quality patient care, cancer research, cancer prevention, and education of oncologists and researchers. NCCC is one of only 41 NCI-designated Comprehensive Cancer Centers in the United States and is the only such center in New Hampshire or Vermont. To learn more about Norris Cotton Cancer Center research, programs, and clinical trials, visit:
http://cancer.dartmouth.edu
The study “Microscale Magnetic Field Modulation for Enhanced Capture and Distribution of Rare Circulating Tumor Cells,”was funded by the National Institutes of Health and National Cancer Institute Cancer Diagnosis Program grant 1RO1CA130070.

