Understanding the strength of immune responses driven by cancer vaccines or therapies that enhance the immune system may become an easier task following the development of a new tool that assesses how immune cells respond to a particular antigen, a compound that induces an immune response.
The test, described in the study “Evaluating frequency and quality of pathogen-specific T cells,” and published in Nature Communications, may be widely used in clinical applications that range from autoimmune diseases or organ transplantation to cancer, allowing for the development of more effective therapies.
The immune system can recognize a huge amount of antigens from targets like viruses, bacterias and cancer cells, against which it develops more- or less-powerful reactions. However, not every immune reaction is the same, and sometimes immune responses are too weak, while others are too strong and damaging. Indeed, in some cases, inappropriate responses to healthy cells or harmless compounds may occur, which often results in auto-immune diseases or allergies.
But until now, researchers had limited means of predicting just how strong an immune reaction would be to a particular antigen. The existing tests were either took too long (one or two days), or weren’t sensitive enough.
Now, Thomas Jefferson University researchers have come up with a new test, CaFlux, that assesses this question.
“The test, which we’ve named the CaFlux, is rapid, sensitive and can test a broad array of antigenic targets,” senior author Yuri Sykulev, MD, PhD, a professor of microbiology and immunology and of medical oncology at the Sidney Kimmel Cancer Center at Jefferson, said in a press release. “A future application of this test could help determine who might respond with a sneeze versus who might respond with anaphylactic shock before it happened,” he said.
The test makes use of one of the first processes that occur when immune cells recognize a target — the opening of calcium channels.
When an immune response takes place, the so-called antigen-presenting cells are the first to come into play. They engulf the pathogen and then present specific proteins from this pathogen at their surface that are to be recognized by killing T-cells. The specificity of the T-cell toward the protein presented by the antigen-presenting cell will determine the strength of the immune response toward that particular antigen. This can be measured by the calcium flux inside the immune cells.
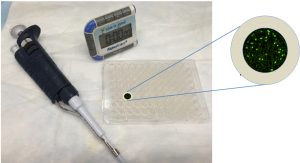
CaFlux works by attaching both antigen-presenting cells and T-cells and then flowing potential antigens to see how the cells react. If the antigen addition induces a strong match between the displayer and responder cell, the two cells grab each other with their receptors, which triggers the opening of the calcium channels. This mechanism then activates a green fluorescence in the cells that can be detected easily under a microscope and quantified using a specific software. The stronger the match, the brighter the green color.
The particularity of this test is that it allows researchers to see not only how many cells respond in a given sample, but also how powerful and how rapid each individual cells responds over time. Combining these three pieces of information may allow for a better understanding of how each person will react to a wide number of immune threats.
In addition, it may lead to the development of better cancer vaccines and immunotherapies, as it allows researchers to first test the potency of such interventions.


