Fate Therapeutics will soon initiate a Phase 1 trial investigating its CAR T-cell therapy, FT819, across several B-cell cancers, including chronic lymphocytic leukemia (CLL), acute lymphoblastic leukemia (ALL), and non-Hodgkin’s lymphoma (NHL).
The announcement follows the clearance of Fate’s investigational new drug (IND) application by the U.S. Food and Drug Administration (FDA). The trial will determine FT819’s maximum tolerated dose, as well as its safety and clinical activity, in an estimated 297 adults with relapsed or refractory CLL, ALL, and NHL. Refractory cancers don’t respond to treatment.
“The clearance of our IND application for FT819 is a ground-breaking milestone in the field of cell-based cancer immunotherapy,” Scott Wolchko, president and CEO of Fate Therapeutics, said in a press release.
The trial will enroll participants for each cancer independently and separately assess the effects of three treatment regimens of increasing doses of FT819. In the first regimen, participants will receive a single dose of the therapy, while in the second, one dose of FT819 will be given alongside interleukin 2 (IL-2), a molecule that is important for T-cell function. In the last regimen, FT819 will be given three times at smaller doses.
For each cancer type and treatment regimen, dose-expansion groups enrolling up to 15 patients may be added to assess the therapy’s clinical activity.
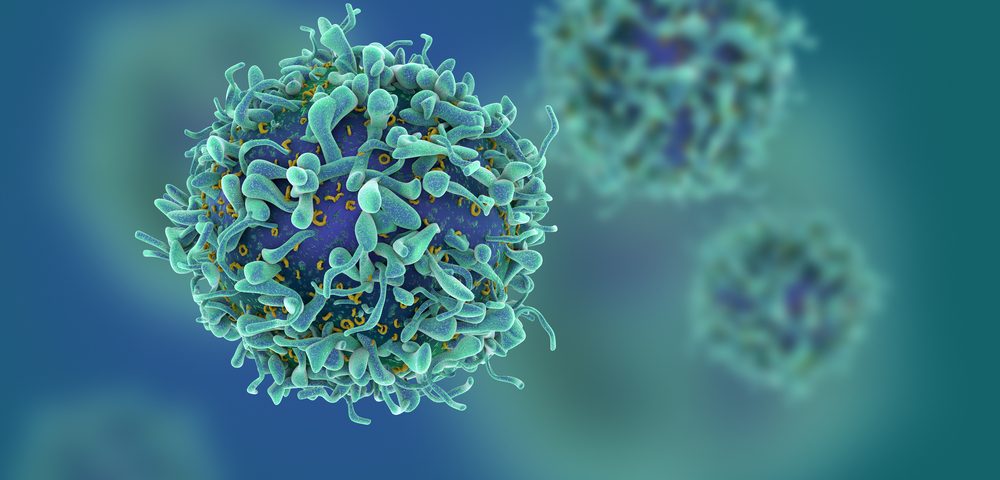
Chimeric antigen receptor T-cell therapy, more commonly known as CAR T-cell therapy, is a type of immunotherapy in which an individual’s T-cells — immune cells with anti-cancer activity — are collected and engineered in the lab to better recognize and eliminate cancer cells. The engineered cells are expanded in the laboratory, and then re-infused in the patient to fight cancer.
FT819 is the first form of CAR T-cell therapy that uses T-cells derived from a line of induced pluripotent stem cells (iPSCs) instead of a human donor. These iPSCs are essentially fully matured cells that were reprogrammed back to a stem-cell state in which they are able to grow into almost any type of cell. This allows modified T-cells to be produced in mass, and delivered off-the-shelf to a large number of patients.
“Our unique ability to produce CAR T cells from a clonal master engineered iPSC line creates a pathway for more patients to gain timely access to therapies with curative potential,” Wolchko said.
Engineered T-cells that make up FT819 also have a series of new features that make them more effective and safer to use. One such feature is the addition of a new receptor, 1XX CAR, that specifically recognizes CD19 proteins to increase their targeted anti-tumor activity without causing exhaustion. Scientists also removed another receptor — the T-cell receptor, or TCR — to lower the risk of graft-versus-host disease (GvHD), a potentially life-threatening complication of conventional CAR T-cell therapy.
Of note, CD19 is a protein found on the surface of malignant B-cells, while GvHD is a complication in which modified T-cells start attacking the host’s body.
Previous preclinical studies of FT819 have shown the therapy has a specificity and cancer cell killing activity comparable to that of donor-derived CAR T-cell therapies. Moreover, FT819’s modified T-cells have been found to remain active and maintain tumor clearance in the bone marrow of an animal model of disseminated lymphoblastic leukemia.
Fate began developing FT819 in 2016 in collaboration with Michel Sadelain, MD, PhD, director of the center for cell engineering at Memorial Sloan Kettering Cancer Center and head of the Gene Expression and Gene Transfer Laboratory.
“Four years ago, we first set out under our partnership with Memorial Sloan Kettering led by Dr. Michel Sadelain to improve on the revolutionary success of patient-derived CAR T-cell therapy and bring an off-the-shelf paradigm to patients, and we are very excited to advance FT819 into clinical development,” Wolchko said.
Under the terms of the agreement established with Memorial Sloan Kettering, Fate holds an exclusive license that covers the therapeutic use of FT819’s modified T-cells. The therapy’s development has been patented (patent 10,370,452) in the U.S., with Sadelain as an inventor.