A new imaging technique can help doctors do a better job of monitoring the immune cells used in cancer immunotherapy by letting them track T-cell movements in the body, according to new research.
The method gives physicians a way to assess immune responses to treatment, and could help researchers understand the processes involved in treatment failure.
Fine-tuned enough to detect when T-cells have found a tumor, the technique also can identify the number of immune cells at a tumor site and the proportion of cells still alive.
The study, “Reporter gene imaging of targeted T cell immunotherapy in recurrent glioma,” was published in the journal Science Translational Medicine.
While immunotherapy has revolutionized the way doctors treat many cancers, the field has a long way to go. Studies show some patients respond remarkably well to CAR T-cell therapy, which involves oncologists removing some of a patient’s T-cells, then re-injecting them after boosting their ability to fight cancer.
A key question that has yet to be answered is why some patients fail to respond to the treatment.
Researchers don’t know if treatment failed because the injected cells wandered off in the wrong direction, if they reached the cancer but were unable to overpower it, or if they died somewhere along the way.
“We are shooting blind,” Sanjiv Gambhir, MD, PhD, professor and chair of radiology at Stanford University School of Medicine, said in a press release. “There are no real tools to see if treatment is working.”
It doesn’t help that it can take months for doctors who rely on measurements of tumor size to recognize that treatment has failed. Sometimes tumors become larger after CAR T-cell therapy because of inflammation triggered by an immune response rather than treatment failure.
Gambhir, the director of the Canary Center at Stanford for Cancer Early Detection and the study’s senior investigator, became aware more than a decade ago of how difficult it is to assess treatment failure.
Back then, he and his colleagues were engineering T-cells that could recognize cancer cells. But they also added what is known as a reporter gene to the cells. The gene makes the cells produce a substance that can be visualized with the help of an imaging method called positron emission tomography (PET).
Since then, the team has worked to improve and test the method — starting with lab animals, and now with humans.
“We can now watch anywhere in your body where those T cells may be,” said Gambhir, who holds the Virginia and D.K. Ludwig Professorship in Cancer Research. “This is the first demonstration in humans of actually noninvasively imaging the immune system in action with reporter gene technology. It’s never been done before in a living human, and without the need to remove any tissue.”
PET scans can show doctors how many T-cells are around a tumor, and if they are alive. Repeating the scans give researchers a way to track the cells as they moved in the body. This can lead to the discovery of new tumors that couldn’t be spotted with standard techniques.
Researchers tested their method in patients with the virulent brain cancer glioblastoma. It showed that T-cells had reached the cancer. But despite this, all the patients died.
The hope is that the method will now provide insight into such treatment failures.
Gambhir’s team said the method can be used far beyond brain cancer.
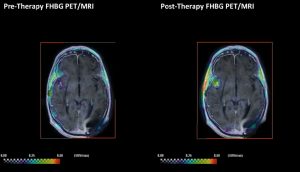
At the moment, the technique has a downside. Although researchers can see T-cells at the site of a cancer, they can not discriminate between cells that have bound to the tumor and those that are idly sitting nearby. But the team is working towards a solution to that problem.
“Right now, the reporter gene is always on,” he said. “But we could change the reporter gene so it only comes on after it latches onto the tumor cell and kills it.” That approach works in mice, but isn’t ready for human trials, he said.